Solid Oxide Fuel Cells (SOFCs) are a cutting-edge clean energy solution designed for high-efficiency, long-duration power generation. Unlike other fuel cells that use liquids or polymers, SOFCs utilize a solid ceramic electrolyte and operate at extremely high temperatures—making them uniquely suited for industrial, stationary, and co-generation applications.
This post explores how SOFCs work, their advantages, limitations, and where they’re used in the real world.
What Is a Solid Oxide Fuel Cell?
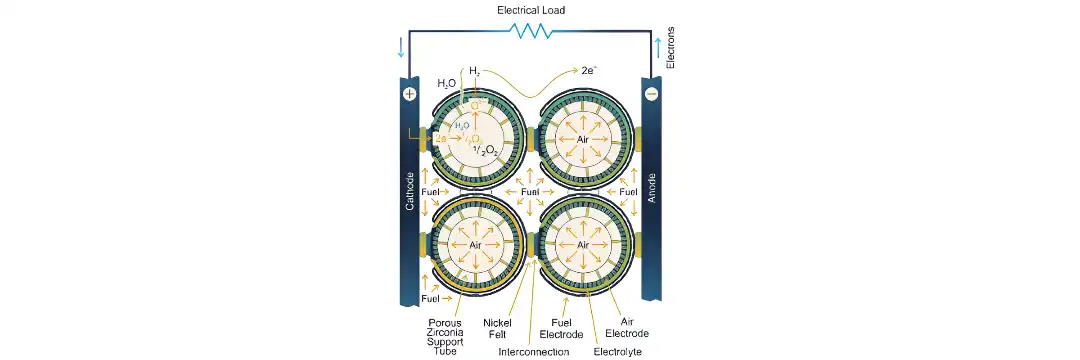
A Solid Oxide Fuel Cell (SOFC) is a high-temperature fuel cell that uses a solid oxide ceramic material to conduct oxide ions (O²⁻) from the cathode to the anode. This ionic flow is the opposite of proton-conducting fuel cells, offering distinct advantages in terms of fuel flexibility and thermal efficiency.
Key Technical Features:
- Electrolyte: Solid zirconia (ZrO₂), typically stabilized with yttria (Y₂O₃) or other rare earth oxides
- Electrodes: Metal oxides or cermets (nickel, cobalt)
- Design Formats:
- Planar (flat): Compact, high power density
- Tubular: Durable, better thermal cycling
- Operating Temperature: ~1000°C (1830°F)
- Operating Pressure: ~1 barg (15 psig)
- Voltage Output per Cell: ~0.8–1.0 VDC
SOFCs are built like computer chips—with thin layers of materials deposited and sealed into stacked modules, making them scalable for various energy needs.
How Do SOFCs Work? The Electrochemistry Explained
The electrochemical process in SOFCs involves oxygen ions traveling through the solid electrolyte from the cathode (air side) to the anode (fuel side), where they oxidize fuel molecules and release electrons.
Anode Reactions (Fuel Side):
- Hydrogen Oxidation
H₂ + O²⁻ → H₂O + 2e⁻ - Carbon Monoxide Oxidation
CO + O²⁻ → CO₂ + 2e⁻
(Occurs when using hydrocarbon fuels)
Cathode Reaction (Air Side):
½O₂ + 2e⁻ → O²⁻
Oxygen from air captures electrons and forms oxide ions.
Overall Reactions:
- Hydrogen:
H₂ + ½O₂ → H₂O - Hydrocarbons:
CO + ½O₂ → CO₂
Unlike PEMFCs and MCFCs, reaction products accumulate at the anode (not the cathode), requiring efficient water and CO₂ removal mechanisms.
Benefits of SOFC Technology
SOFCs offer a powerful combination of efficiency, flexibility, and resilience, making them ideal for stationary energy needs and cogeneration.
Top Advantages:
| Feature | Description |
| Internal Fuel Reforming | Converts methane or other light hydrocarbons directly into hydrogen inside the cell—no need for external reformers |
| High Efficiency | Electrical efficiency of ~60%, rising to ~85% in CHP systems |
| Fuel Versatility | Compatible with hydrogen, natural gas, biogas, and syngas |
| Cogeneration-Ready | Produces high-quality waste heat for use in district heating or industrial processes |
| No Noble Metals Needed | Uses nickel-based catalysts instead of costly platinum or palladium |
| Durable Solid-State Design | Avoids electrolyte leakage and supports diverse geometries (tubular, planar) |
Challenges & Limitations of SOFCs
Despite their technical promise, SOFCs face several material and manufacturing constraints that impact scalability and cost.
Primary Challenges:
| Limitation | Impact |
| Material Stress at High Temperatures | Components must withstand 1000°C+ continuously without cracking, degrading, or expanding |
| Thermal Cycling Sensitivity | Rapid heating and cooling can cause delamination or fracture between layers |
| 🛠️Complex Fabrication | Requires precision layering and sealing; current manufacturing is not yet cost-efficient at scale |
| Sulfur Tolerance | Can tolerate more sulfur than MCFCs (~50 ppm), but still sensitive to H₂S and COS, which reduce performance |
| Immature Ecosystem | Still under development; lacks the commercial maturity and supply chain support of PEMFCs or batteries |
Where Are SOFCs Used?
SOFCs are not designed for cars or mobile devices. Instead, they excel in long-duration, high-load, and stationary applications.
Common Applications:
- Commercial & Industrial CHP (Combined Heat and Power)
- Off-grid & Remote Energy Systems
- Backup Power for Data Centers & Hospitals
- Biogas & Waste-to-Energy Plants
- Rail and Marine Propulsion (emerging)
SOFCs vs Other Fuel Cells: Quick Comparison
| Feature | SOFC | MCFC | PEMFC |
| Electrolyte Type | Solid zirconia ceramic | Molten carbonates | Polymer membrane |
| Ion Conducted | O²⁻ | CO₃²⁻ | H⁺ |
| Operating Temp | ~1000°C | ~650°C | ~80°C |
| Fuel Flexibility | High | High | Low (pure hydrogen only) |
| Startup Time | Long | Long | Short |
| Use Case | Industrial CHP, grids | Utility, power stations | Transport, mobile |
| Maturity | Emerging | Mid | Commercialized |