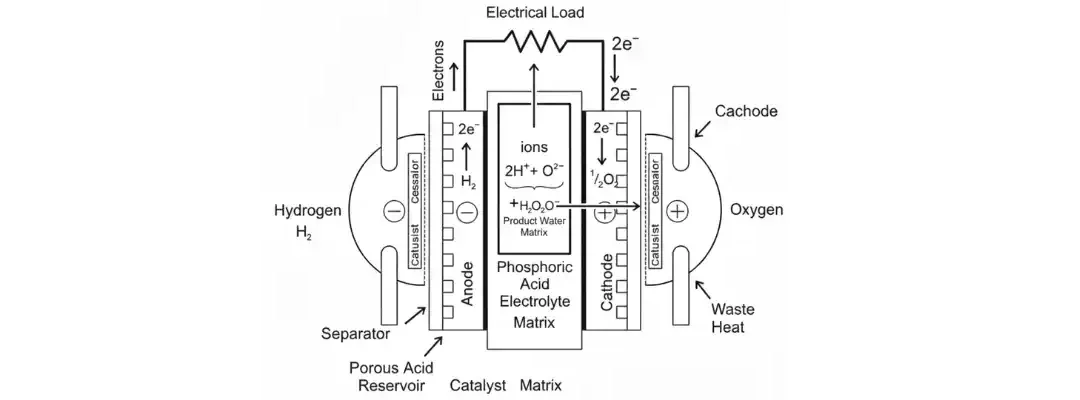
Phosphoric Acid Fuel Cells (PAFCs) are one of the most established types of fuel cells, known for their stable performance, moderate temperature operation, and use in commercial and stationary power systems. PAFCs are well-suited for combined heat and power (CHP) due to their ability to produce both electricity and usable heat.
This post explores how PAFCs work, their benefits and challenges, and where they are used in real-world applications.
What Is a Phosphoric Acid Fuel Cell?
A Phosphoric Acid Fuel Cell uses liquid phosphoric acid as the electrolyte, which conducts hydrogen ions (H⁺) from the anode to the cathode. The acid is held in place within a silicon carbide matrix, which helps contain and support the electrolyte during operation.
Operating Characteristics
| Parameter | Specification |
| Electrolyte | Liquid Phosphoric Acid |
| Ion Conducted | H⁺ (Hydrogen ions) |
| Operating Temp | 150–205°C (300–400°F) |
| Operating Pressure | ~15 psig (1 barg) |
| Voltage per Cell | Up to 1.1 VDC |
| Typical Fuel | Hydrogen or Hydrogen-rich Reformate |
| Oxidant | Air (CO₂ scrub not required) |
How Do PAFCs Work?
Anode Reaction (Fuel Side):
H₂ → 2H⁺ + 2e⁻
Hydrogen splits into protons and electrons. The protons (H⁺) move through the electrolyte to the cathode.
Cathode Reaction (Air Side):
½O₂ + 2H⁺ + 2e⁻ → H₂O
Oxygen from air reacts with the protons and electrons to form water at the cathode.
Overall Cell Reaction:
H₂ + ½O₂ → H₂O
The main by-product is water, which collects at the cathode and must be removed regularly.
Benefits of PAFC Technology
Phosphoric Acid Fuel Cells are a strong choice for stationary systems that need reliable, continuous power.
Advantages:
- CO₂ Tolerant
Handles up to 30% CO₂ – no need for purified oxygen or air scrubbing. - Produces Usable Heat
Waste heat can be recovered for heating buildings or water (ideal for co-generation). - Stable Electrolyte
Phosphoric acid remains effective at high temps with low evaporation. - Runs on Reformate Fuel
Can use hydrogen-rich gas mixtures from reformers.
Advantages of PAFC Technology
Phosphoric Acid Fuel Cells offer a practical balance between performance, stability, and co-generation capability:
| Advantage | Benefit |
| CO₂ Tolerance | Handles up to 30% CO₂ – allows use of regular air and reformate fuel |
| Useful Heat Output | High-grade waste heat suitable for CHP (Combined Heat & Power) |
| Stable Electrolyte | Low volatility, even at higher temperatures |
| Simple Design | Does not require ultra-pure fuel or oxidant compared to other low-temp cells |
| Commercial Proven | Used in stationary power applications globally |
Applications of PAFCs
PAFCs are best used in stationary power setups, especially when heat recovery is important.
Common Use Cases:
- Hospitals and Clinics – Reliable power for critical systems
- Commercial Buildings – Provides electricity + heating
- Off-Grid Power Systems – Where air quality can’t be strictly controlled
- Combined Heat and Power (CHP) plants
PAFC vs Other Fuel Cells
| Feature | PAFC | PEMFC | SOFC |
| Electrolyte | Liquid Phosphoric Acid | Solid Polymer Membrane | Solid Oxide Ceramic |
| Temp Range | 150–205°C | 60–100°C | ~1000°C |
| Fuel Flexibility | Moderate | Low | High |
| Startup Time | Slow | Fast | Very Slow |
| Heat Output | Medium (CHP ready) | Low | High (co-generation) |
| Mobile Use | No | Yes | No |