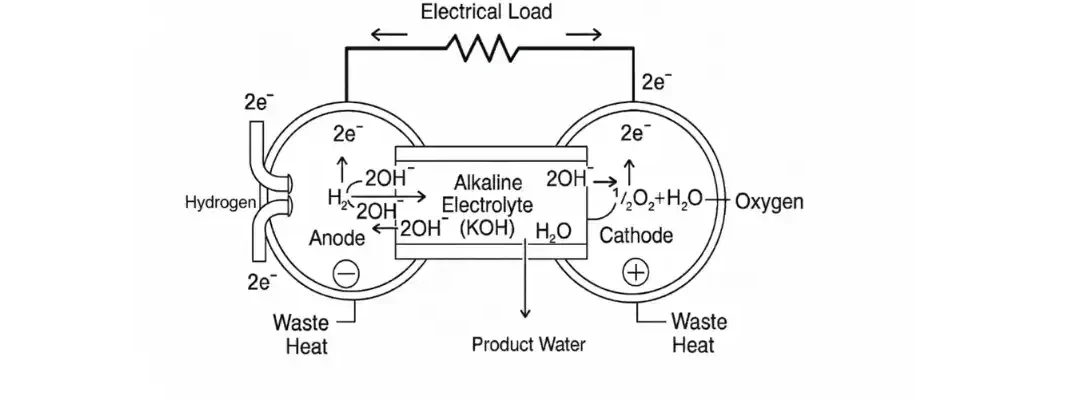
Alkaline Fuel Cells (AFCs) are among the oldest types of fuel cells—and still one of the most efficient for converting hydrogen and oxygen into electricity. They’re compact, lightweight, and ideal for situations where space and energy efficiency matter most.
Used by NASA during the Apollo space missions, AFCs are trusted technology for high-performance environments. But they also come with limitations that affect their use in everyday settings.
Let’s break down how AFCs work, their pros and cons, and where they’re most useful.
What Is an Alkaline Fuel Cell?
An Alkaline Fuel Cell uses a special liquid (usually potassium hydroxide, or KOH) as the electrolyte. This liquid moves hydroxyl ions (OH⁻) from the air side (cathode) to the fuel side (anode)—where electricity is produced.
Two Types of AFC Designs
- Mobile Electrolyte AFCs
1.Liquid KOH circulates between the electrodes.
2.Water and heat are removed as the fluid flows. - Immobile Electrolyte AFCs
1.A thick KOH paste is held in a solid matrix.
2.Water evaporates into the hydrogen stream and is later condensed.
Operating Characteristics
| Parameter | Value |
| Operating Temp | 65–220°C (150–430°F) |
| Operating Pressure | ~15 psig (1 barg) |
| Voltage per Cell | ~1.1 to 1.2 VDC |
| Electrolyte | Potassium Hydroxide (KOH) |
Electrochemical Reactions in AFCs
Anode Reactions:
H₂+ 2K⁺+ 2OH⁻→ 2K + 2H₂O2K → 2K⁺+ 2e⁻
These reactions release electrons and form water at the anode side.
Cathode Reactions:
½O₂+ H₂O → 2OH2OH + 2e⁻→ 2OH⁻
Oxygen reacts with water to form hydroxyl ions, which migrate to the anode.
Overall Cell Reaction:
H₂+ ½O₂→ H₂O
In both mobile and immobile systems, water is the by-product, but its removal method varies based on the electrolyte type.
Advantages of Alkaline Fuel Cells
Despite being an older technology, AFCs offer several compelling advantages in the right environment:
| Benefit | Description |
| Low Operating Temperature | Ideal for portable and space-based systems |
| High Electrical Efficiency | Converts more fuel into electricity (up to 60%) |
| Fast Startup | Can reach 50% power at ambient temperature—great for quick deployment |
| Minimal Platinum Requirement | Reduces cost vs. PEM fuel cells |
| Low Corrosion Risk | Chemically stable under operating conditions |
| Compact & Lightweight | Ideal for aerospace and low-weight applications |
| Simple System Design | Easier to integrate and operate |
Key Features at a Glance
| Feature | Detail |
| Temperature | 150–430°F (65–220°C) |
| Pressure | ~15 psi |
| Voltage per Cell | 1.1–1.2 VDC |
| Fuel Type | Pure Hydrogen |
| Oxidant | Pure Oxygen or CO₂-free air |
Why Choose Alkaline Fuel Cells?
Top Advantages:
- Fast startup – reaches 50% power at room temp
- High efficiency – more energy from less fuel
- No expensive platinum needed
- Low temperature operation
- Simple and lightweight system
- Low corrosion risk – longer component life
Summary Table: Alkaline Fuel Cells (AFCs)
| Feature | Alkaline Fuel Cells (AFCs) |
| Electrolyte | Potassium Hydroxide (KOH) – mobile or paste-based |
| Ion Conducted | Hydroxyl Ion (OH⁻) |
| Operating Temp | 65–220°C (150–430°F) |
| Fuel Type | Pure Hydrogen |
| Oxidant Requirement | Pure Oxygen or CO₂-free Air |
| Startup Time | Fast – 50% power at ambient temp |
| Efficiency | High (up to 60%) |
| CO₂ Tolerance | Very Low (max ~350 ppm) |
| Best Use Cases | Space missions, portable systems, lab-scale research |