As clean energy technologies evolve, fuel cells and batteries are at the forefront of powering everything from smartphones to spacecraft. While they’re both electrochemical devices, they work in fundamentally different ways.
In this guide, we’ll break down:
- How fuel cells and batteries work
- Key structural and operational differences
- Real-world applications
- When to use one over the other
Fuel Cells and Batteries:
Both fuel cells and batteries are galvanic cells—they generate electrical energy through electrochemical reactions. Here’s what they share:
Similarities:
- Both have an anode, cathode, and electrolyte
- Both produce direct current (DC)
- Both force electrons through an external circuit to do work
- Both require multiple cells in series to deliver higher voltages
That’s where the similarities end.
Key Differences: Fuel Cells vs. Batteries
Here’s a side-by-side comparison to help you quickly understand how they differ:
| Feature | Fuel Cells | Batteries |
| Energy Input | Supplied externally (e.g. hydrogen, oxygen) | Stored internally within the device |
| Electrode Materials | Gases (H₂, O₂) + platinum catalyst | Solid metals (e.g. lithium, zinc) and metal oxides |
| Energy Supply Duration | Continuous, as long as fuel is supplied | Finite, until chemicals are depleted |
| Rechargeability | No recharge needed, just refuel | Requires recharging or replacement |
| Scalability | Ideal for large systems or long-duration power | Better for small, portable devices |
| Efficiency Loss | Minimal, unless fuel interrupted | Capacity degrades over time with cycles |
| Environmental Impact | Zero-emissions if green hydrogen is used | Varies depending on material and disposal methods |
Energy Source & Storage Method
- Batteries are closed systems: their chemical reactants are stored inside. When depleted, they must be recharged or discarded.
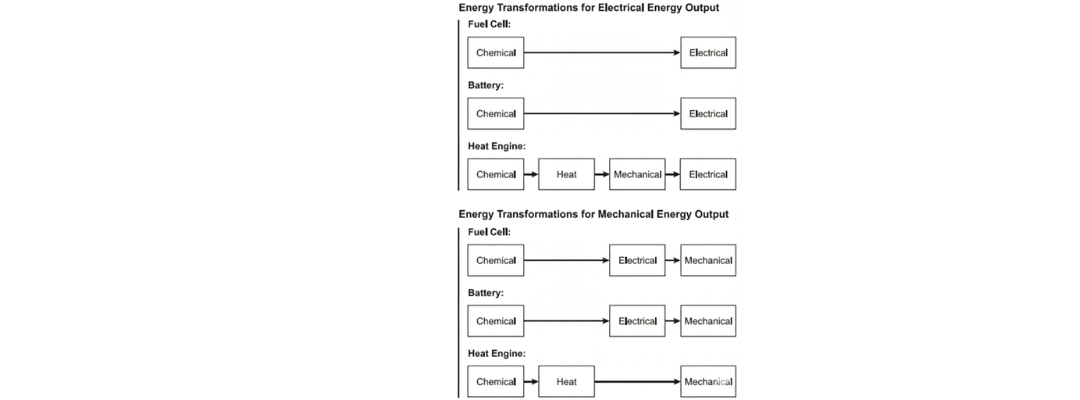
- Fuel cells are open systems: they receive reactants from external sources, such as hydrogen and oxygen tanks. They operate continuously as long as the fuel is supplied and waste is removed.
This key difference makes fuel cells more suited for continuous, long-duration applications.
Electrode Function: Solid vs. Gas-Based Chemistry
- In batteries, both the anode and cathode are solid materials, such as lithium and cobalt oxide.
- In fuel cells, the electrodes interact with gaseous reactants (hydrogen at the anode, oxygen at the cathode), often with a platinum catalyst to speed up reactions.
This means fuel cells have fewer moving parts and can achieve cleaner energy conversion.
Electrochemical Reaction Flow
Here’s a simplified breakdown of what happens inside:
Battery Reaction:
- Internal reactants convert chemical energy to electricity.
- Once depleted, recharge is needed.
- Efficiency decreases over time with usage cycles.
Fuel Cell Reaction:
- External hydrogen is split into protons and electrons at the anode.
- Electrons travel through an external circuit, powering devices.
- Protons move through the electrolyte to combine with oxygen at the cathode, forming water.
- The reaction continues as long as fuel is supplied.
Real-World Applications: When to Use What
Batteries are best for:
- Smartphones
- Laptops
- Electric cars (short range)
- Home solar storage systems
🛠️ Fuel cells excel at:
- Hydrogen-powered vehicles (e.g., Toyota Mirai, Hyundai NEXO)
- Drones and long-range mobility
- Backup power in hospitals or data centers
- Space missions (NASA uses fuel cells on spacecraft)
Environmental Impact and Sustainability
- Fuel cells, when powered by green hydrogen, produce zero emissions—just water vapor as a by-product.
- Batteries can have a larger environmental footprint, especially due to lithium mining, heavy metals, and disposal issues.
Expert Insight: Which Is the Future?
Fuel cells and batteries aren’t in competition—they complement each other.
- Use batteries where compact, rechargeable energy is needed.
- Use fuel cells where long-range, high-power, or continuous operation is required.
Together, they form a hybrid ecosystem that powers the transition to renewable energy.