Galvanic cells are at the heart of technologies that power everything from portable electronics to electric vehicles. These electrochemical cells transform chemical energy into electrical energy through spontaneous redox reactions. In this blog, we explore how galvanic cells function, the essential components involved, their practical design, and their importance in the transition to clean energy.
What Is a Galvanic Cell?
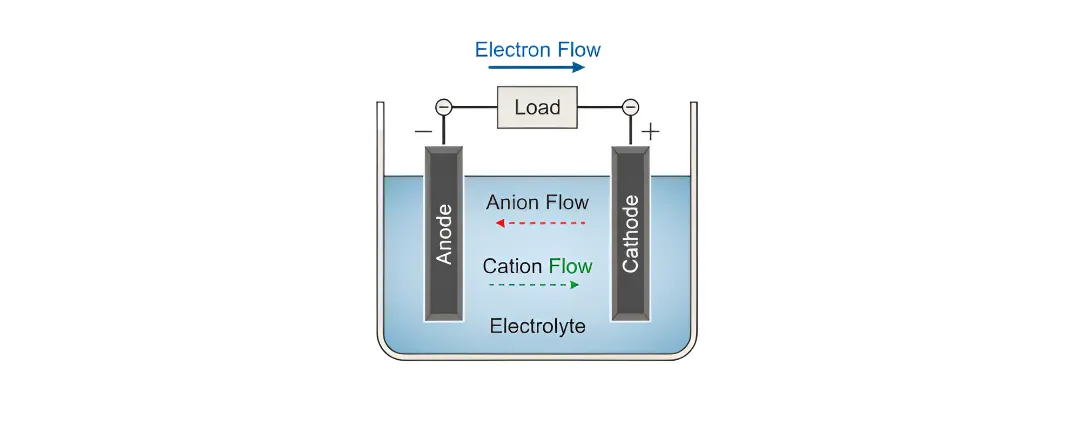
A galvanic cell (also called a voltaic cell) is a device that produces electricity through a spontaneous chemical reaction. It is the principle behind everyday batteries and advanced fuel cell systems. These cells consist of:
- Anode (negative electrode): Site of oxidation — releases electrons
- Cathode (positive electrode): Site of reduction — accepts electrons
- Electrolyte: Ion-conducting medium that separates the electrodes and supports ion flow
Together, these elements create an electrochemical environment where chemical energy is efficiently converted to electrical energy.
How Does a Galvanic Cell Work?
1. Separation of Electrodes to Capture Energy
When the anode and cathode are directly connected, a reaction may occur, but it simply releases heat—no electricity. By separating the electrodes and connecting them through an external load, electrons are forced to travel through that load, performing useful work (e.g., lighting a bulb).
2. Electron and Ion Movement
- Electrons flow from anode cathode via an external circuit
- Cations and anions move within the electrolyte to balance charge
This controlled transfer generates a steady electric current.
3. Sustaining the Redox Reaction
The chemical process continues as long as:
- The circuit remains closed
- Reactants are available
- Ionic conduction is maintained in the electrolyte
As the reaction proceeds, the cell develops a voltage difference called open-circuit voltage (OCV). This value depends on the materials used and the operating conditions.
The Role of the Electrolyte
The electrolyte is central to galvanic cell functionality. It must:
- Conduct ions (but not electrons)
- Be chemically stable with electrodes
- Operate across wide temperature ranges
Example: When table salt (NaCl) dissolves in water, it forms Na⁺ and Cl⁻ ions, enabling ion flow and enhancing conductivity. This ion migration is key to the redox reaction’s continuity.
Chemical Reactions Inside the Cell
At the Anode:
- Oxidation occurs (loss of electrons)
- Electrons build up, giving it a negative charge
At the Cathode:
- Reduction occurs (gain of electrons)
- Attracts cations and becomes positively charged
Completing the Circuit:
Electrons travel through an external load, re-enter the cell at the cathode, and complete the chemical transformation. This continues until:
- The anode material is depleted
- The electrolyte fails
- The external circuit is broken
Reaction Products:
Depending on the chemistry, products may be:
- Solids (e.g., metal deposits)
- Liquids
- Gases
These by-products must be managed to maintain electrode efficiency.
Practical Design Considerations
Porous Barrier or Salt Bridge
A porous membrane or salt bridge is often placed between electrodes. This design:
- Permits ion flow
- Prevents electrode contact
- Maintains reaction stability
Electrolyte Types
Electrolytes can be:
- Liquid: Acidic or basic solutions (e.g., sulfuric acid in lead-acid batteries)
- Solid: Polymer or ceramic electrolytes (used in solid-state batteries)
The choice of electrolyte directly affects:
- Power output
- Efficiency
- Safety and lifespan
Why Galvanic Cells Matter in Clean Energy
Galvanic cells enable clean, combustion-free electricity generation, making them essential for:
- Consumer batteries (AA, lithium-ion, etc.)
- Fuel cells for electric vehicles and backup power
- Remote and portable energy systems
They offer advantages such as:
- High energy density
- Silent operation
- Modularity and scalability
As we move toward decarbonization, galvanic and fuel cells will play a pivotal role in replacing fossil-fuel-based systems.