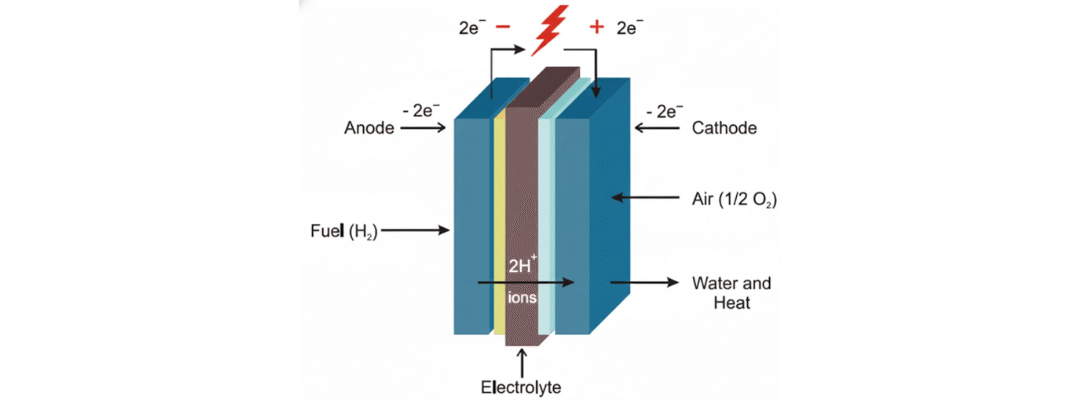
Fuel cells are an advanced clean energy technology, capable of powering everything from vehicles to industrial plants. But not all fuel cells are created equal. The type of electrolyte used inside a fuel cell determines how it operates, what fuels it can use, what temperatures it can tolerate, and where it’s best applied.
This guide dives deep into the major types of fuel cells, categorized by temperature and electrolyte composition, with insights into their advantages, challenges, and ideal use cases.
Why Electrolyte Type Matters
The electrolyte inside a fuel cell determines:
- Which ions are transferred (e.g., H⁺, O²⁻, OH⁻)
- The required operating temperature
- The need for catalysts and their cost
- The type of fuel input (pure hydrogen or hydrocarbons)
- Startup behavior and mechanical durability
High-Temperature Fuel Cells
Operating Temp: Typically above 600°C (1112°F)
High-temperature fuel cells have the ability to perform internal reforming, converting hydrocarbon fuels such as methane into hydrogen and carbon monoxide within the cell itself. This is made possible through steam reforming at the anode, often using a nickel catalyst.
Key Benefits:
- Fuel flexibility – can use natural gas, biogas, or methane
- Internal reforming – no need for separate reformer unit
- High efficiency – up to 65% in electricity generation, and 85% in CHP (Combined Heat and Power) systems
- Co-generation ready – valuable high-grade waste heat
- No platinum needed – uses non-noble metal catalysts
Types:
1. Solid Oxide Fuel Cell (SOFC)
- Electrolyte: Solid ceramic (usually yttria-stabilized zirconia)
- Ion conducted: O²⁻ (oxygen ions)
- Ideal for: Utility-scale, industrial CHP systems, high-temperature environments
2. Molten Carbonate Fuel Cell (MCFC)
- Electrolyte: Molten carbonate salts
- Ion conducted: CO₃²⁻ (carbonate ions)
- Ideal for: Large-scale power plants, high-efficiency electricity + heat production
Low-Temperature Fuel Cells
Operating Temp: Below 250°C (482°F)
Low-temperature fuel cells require pure hydrogen because they can’t perform internal fuel reforming. They are known for their fast startup, compact size, and compatibility with mobile or transport applications.
Key Benefits:
- Fast startup – ideal for automotive use
- Compact and lightweight
- Easier to integrate into vehicles
- Lower operating stress on materials
- Better for stop-start cycles
Types:
1. Proton Exchange Membrane Fuel Cell (PEMFC)
- Electrolyte: Solid polymer membrane (e.g., Nafion)
- Ion conducted: H⁺ (protons)
- Ideal for: Cars (Toyota Mirai, Hyundai NEXO), forklifts, drones
2. Alkaline Fuel Cell (AFC)
- Electrolyte: Aqueous potassium hydroxide (KOH)
- Ion conducted: OH⁻ (hydroxide ions)
- Ideal for: Spacecraft (NASA use), military-grade equipment
3. Phosphoric Acid Fuel Cell (PAFC)
- Electrolyte: Liquid phosphoric acid soaked in a matrix
- Ion conducted: H⁺
- Ideal for: Stationary power generation in hospitals, hotels, commercial buildings
Summary Table: Fuel Cell Types at a Glance
| Type | Electrolyte | Temp Range | Ion Type | Fuel Type | Common Applications |
| SOFC | Solid oxide ceramic | 600–1000°C | O²⁻ | Natural gas, methane | Industrial power, CHP |
| MCFC | Molten carbonate salts | ~650°C | CO₃²⁻ | Biogas, fossil fuels | Utility-scale generation |
| PAFC | Phosphoric acid | ~200°C | H⁺ | Hydrogen | Commercial buildings, hospitals |
| AFC | Aqueous KOH | ~60–90°C | OH⁻ | Pure hydrogen | Aerospace, military |
| PEMFC | Polymer membrane | ~80°C | H⁺ | Hydrogen | Vehicles, mobile devices |
How to Choose the Right Fuel Cell Type
| Application | Recommended Fuel Cell Type | Why? |
| Electric Vehicles | PEMFC | Fast startup, low temp, compact |
| Industrial CHP | SOFC / MCFC | High-grade heat, fuel flexibility |
| Portable Electronics | PEMFC or AFC | Lightweight, efficient |
| Off-grid Power | SOFC / PAFC | Durable, long-term supply |
| Aerospace | AFC | Proven history in space systems |